Index
- Jorney
- Creation of Anvisa
- International Actions
- Anvisa Structure
- How Anvisa works
- Regulatory Process
- Strategic Projects
Jorney
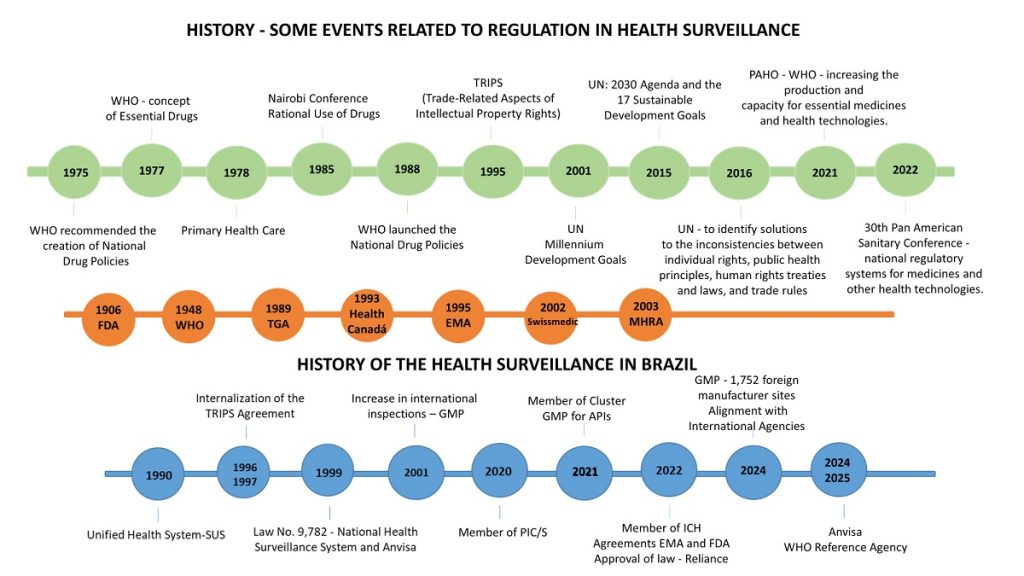
Some events related to regulation in Health Surveillance
1975 – WHO/28th Assembly: recommended the creation of national drug policies.
1977 – WHO: discussed the concept of essential drugs.
1978 – Declaration of Alma Ata/USSR: Primary Health Care.
1985 – Nairobi Conference: established the terminology of rational use of drugs.
1988 – WHO launched the publication entitled Guidelines for developing national drug policies, renamed as How to develop and implement a National Drug Policy.
1995 – change in the International Intellectual Property System, with approval of TRIPS (Agreement on Trade-Related Aspects of Intellectual Property Rights), and the creation of the World Trade Organization (WTO): strengthening the competitiveness of innovative companies by establishing the recognition of patents in all technological sectors.
2001 – UN: Millennium Development Goals.
2015 – UN: 2030 Agenda and the 17 Sustainable Development Goals – providing access to quality medicines and vaccines was considered essential to the right to health.
2016 – High-Level Panel of the United Nations Secretary-General: to identify solutions to the inconsistencies between individual rights, public health principles, human rights treaties and laws, and trade rules.
2021 – 59th Session of the Directing Council of PAHO and the WHO Regional Committee for the Americas: increasing the production capacity for essential medicines and health technologies.
2022 – 30th Pan American Sanitary Conference: health agenda for the coming years, including a resolution on policy to strengthen national regulatory systems for medicines and other health technologies.
Brazil´s impact on the Global Stage
- In 1995, at the 48th WHA: concern about the negative impact on local production in developing countries with the entry into force of the WTO TRIPS Agreement.
- In 2001 – HIV and AIDS pandemic: Millions of people living with HIV died in the developing countries most affected by HIV without access to effective medications and treatment regimens (OLIVEIRA & ESHER, 2004).
- Brazil – more than 100,000 Brazilians living with HIV received antiretroviral therapy (ART).
- Since 1996, the National HIV/AIDS Control Program has provided universal treatment. In Brazil, the lack of patent protection for pharmaceutical products and processes until 1997, combined with the country’s ability to develop the first antiretrovirals (ARVs) via reverse engineering, as well as to produce them locally, led to a significant reduction in the costs of ART (antiretroviral therapy) and enabled the provision of medicines from the National HIV and AIDS Program (PNAIDS) to a growing number of patients (OLIVEIRA & SANTOS, 2016).
- Another measure adopted by the Brazilian government: issuing a single compulsory license in 2007 for Efavirenz, used in 40% of therapies and compromising 70% of PNAIDS’ financial resources.
- In 2001, at the United Nations General Assembly’s special session on HIV/AIDS (UNGASS, 2001), it was recognized that prevention, health care and drug treatment were mandatory conditions for the effective fight against the pandemic.
- In Doha, Qatar, the WTO Ministerial Conference included the issue of access to medicines in the context of the TRIPS Agreement and approved the Declaration on the TRIPS Agreement and Public Health (WTO, 2001), which states that nothing in the TRIPS Agreement should prevent member countries from adopting measures, including the use of all flexibilities in the agreement, to protect public health.
History of the Pharmaceutical Sector in Brazil
- 1890 – 1909: 208 pharmaceutical industries. A few years later, this number reached 455.
- In 1920, the pharmaceutical industry ranked fourth among the most important industrial sectors in the country, with 8% of total production, behind the food industry (40%), textiles (28%) and clothing (8.2%) (Sindusfarma, 2018).
- 1900 – 1910: laboratories from Europe and the United States accounted for 2.1% of the market and, in 1920, reached 7.3% of the national pharmaceutical market’s revenue (Sindusfarma, 2018; Tigre, 2016).
- 1990 – Implementation of the Unified Health System (SUS), with free and universal care.
- 1996-1997: Commercial opening of the country and internalization of the TRIPS Agreement.
- 1999 – Law No. 9,782, which defines the National Health Surveillance System and creates the National Health Surveillance Agency – Anvisa.
- 1999 – Law No. 9,787 – establishes generic medicines in the country.
1999 – Creation of Anvisa
National Health Surveillance System – SNVS (¹)
- Anvisa: coordination of the SNVS.
- 27 state health surveillance agencies.
- 5,561 municipal health surveillance services.
- 01 laboratory – National Institute for Quality Control in Health (INCQS/Fiocruz).
- 23 Official Laboratories – production and research – food, medicines, immunobiologicals, blood derivatives (State, Navy, Air Force and Army).
- 304 college education institutions – 120 federal, 133 state and 59 municipal, distributed in: university, university centers, colleges and institutes and centers of technological education. (²)
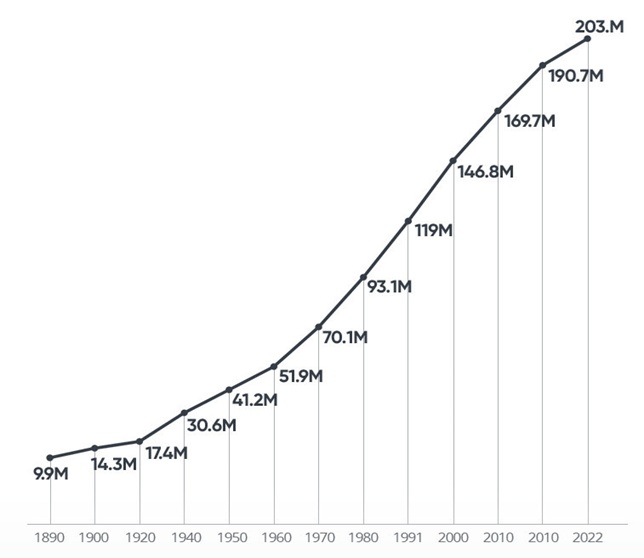
Population of Brazil (³)
- Anvisa: responsible for more than +25% Economy of the Country.
- Grouwth of metropolitan regions
- Increased demand for health services
- Need to improve the health surveillance structure over the next 30 years
(¹) Anvisa; (²) Ministry of Education; (³) IBGE
Anvisa – Operating Structure X Populations of the States

Brazil & connections with PAHO and OECD
PAHO – Sustainable mobility in Brazilian cities, 2019
OECD – Sustainable and Balanced Growth, 2022

(²) OECD (2022), Regulatory Reform in Brazil, OECD Reviews of Regulatory Reform,
OECD Publishing, Paris, https://doi.org/10.1787/d81c15d7-en
Governance Structure
BOARD OF DIRECTORS (DICOL)
- Directors are appointed by the Presidency of the Republic and approved by the National Congress.
- 4-year terms.
- 1st Board (CEO) – Antônio Barra Torres
- Term: Nov 04/2020 to Dec 21/2024
- 2nd Board – Meiruze Sousa Freitas
- Term: Nov 03/2020 to Dec 12/2024
- 3rd Board – Daniel Meirelles Fernandes Pereira
- Term: Aug 15/2022 to Jul 24/2027
- 4th Board – Rômison Rodrigues Mota
- Term: Jul 2021 to Dec 19/2025
- 5th Board – Frederico Augusto de Abreu Fernandes (substitute and interim director)
- Term: Aug 28/2024 to Mar 12/2025
Governance

Anvisa – From Strategy to Execution
How Anvisa works
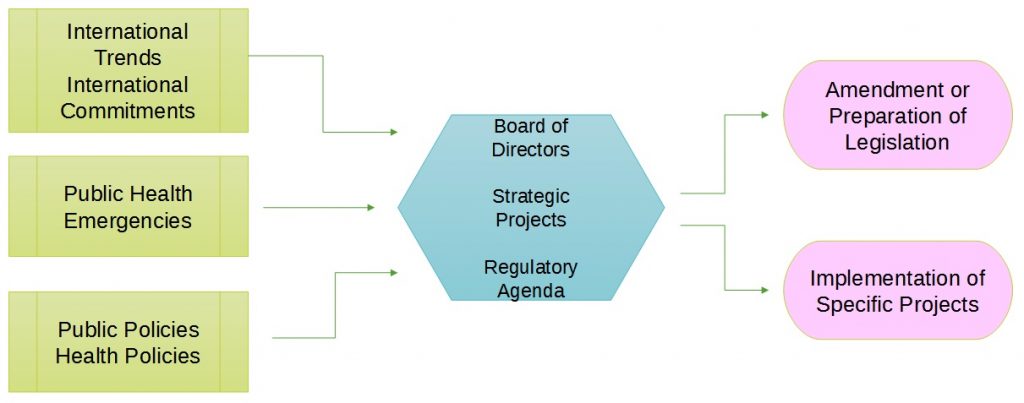
How Anvisa works

Strategic Projects – Multi-Year Plan – PPA
- The Medium-Term Planning Act, established by the 1988 Constitution,
serves as a vital instrument for the Brazilian government. - The PPA sets forth fundamental guidelines, objectives, and targets for capital expenditures and associated outlays within the federal public administration.
- This comprehensive framework provides strategic direction for the allocation of public resources across various sectors, encompassing pivotal areas such as health, education, and infrastructure.
- The PPA 2024-2027 is structured around three core axes: social, economic, and environmental, presenting indicators and programs that align with these dimensions.

Call for comments: Regulatory Impact Assessment (RIA)

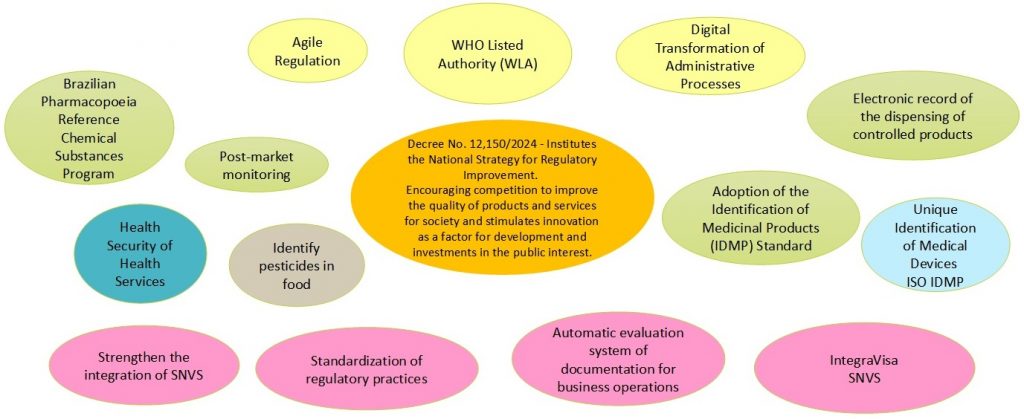
Strategic Framework

Strategic Framework & Regulatory Agenda
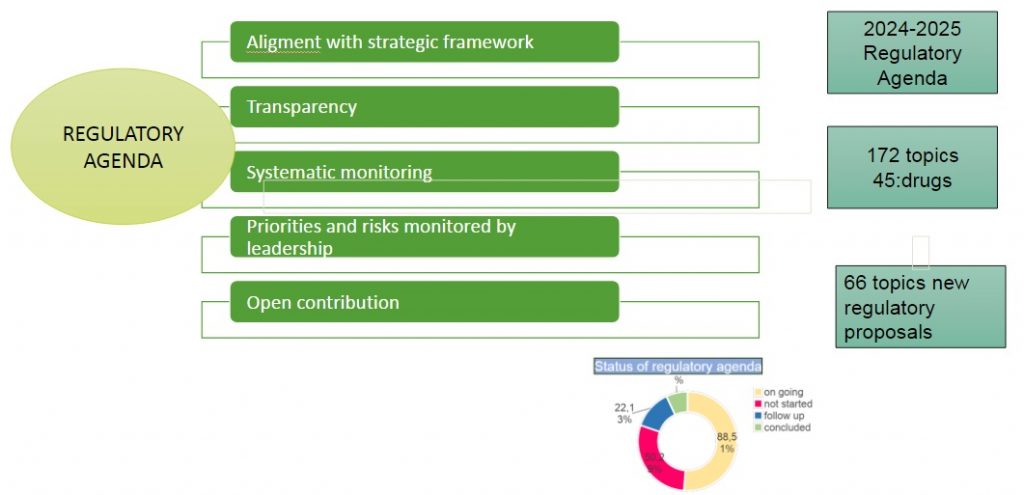
Strategic Framework 2024-2027
Strategic Framework
Agile Regulation – Sandbox
Agile Regulation
Objective: to support the Agency in the complex and dynamic regulatory demands arising from the advancement of emerging technologies.
References: Organization for Economic Cooperation and Development (OECD) and the World Economic Forum.
– Adoption of experimental regulatory approaches, such as the Regulatory Sandbox, and the construction of automated tools for monitoring the performance of the Agency’s regulatory acts.
Regulatory Sandbox
✓ Regulatory environment with flexible normative rules to allow companies to explore new technologies and solutions.
✓ Anvisa and companies can learn more about innovations, generating evidence for making the best regulatory decision.
✓ Accelerates the arrival of new ideas to the market.
✓ Ensures that the products or services tested meet the standards required by the Agency.
Projects approved by Anvisa
- Public Call 1/2024 – Pilot project for regulatory evaluation of herbal medicine, new synthetic medicine and biological product of interest in health services in Brazil.
- Digital Package Insert – Approved in July/2024, which will be valid until December 31, 2026. It involves: Free sample packaging of medicines. Medicines intended for health establishments, except pharmacies and drugstores. Over-the-counter medicine (OTC), sold in multiple packages. Medicines intended for government use, packaged in packaging bearing the Ministry of Health’s own government brands.
Click here to download the pdf article file
Haramefá – Contact
Bento Corrêa
Regulatory Planning and International Cooperation
Send email
Cel: +5561991460021
+556133655423 | +556133655423
SHN Quadra 2 Bloco F
Edificio Executive Office Tower – 1205
Asa Norte – Brasília/DF – Brasil
Zipcode: 70702-000
